
pH adjustment is a critical process in water treatment. Sodium carbonate (Na₂CO₃), also known as soda ash, is widely used as an alkaline agent in industrial wastewater, drinking water pretreatment, and municipal sewage treatment. Following the correct steps ensures efficiency, cost control, and safe operation.
√ Stable and mild alkalinity: Effective for raising acidic pH.
√ Safe handling: Less corrosive compared to caustic soda.
√ Cost-effective: Lower cost in storage and transportation.
Industrial Wastewater
Neutralizes acidic effluent in paper, textile, and chemical industries.
Drinking Water Pretreatment
Adjusts raw water pH, reduces corrosion, and enhances coagulant efficiency.
Agricultural and Farming Wastewater
Stabilizes acidic wastewater in livestock and irrigation systems.
1. Test water quality: Measure the initial pH using a pH meter or test strips.
2. Calculate dosage: Estimate based on acidity and volume, then confirm with lab tests.
3. Prepare solution: Dissolve solid sodium carbonate in water to make a 5–10% solution.
4. Slow dosing: Add the solution gradually to prevent local pH spikes.
5. Mix thoroughly: Use stirring or circulation to ensure even distribution.
6. Recheck pH: Verify the pH level after adjustment, target usually 6.5–8.5.
7. Fine-tune: Adjust the dosage slightly if needed to achieve optimal performance.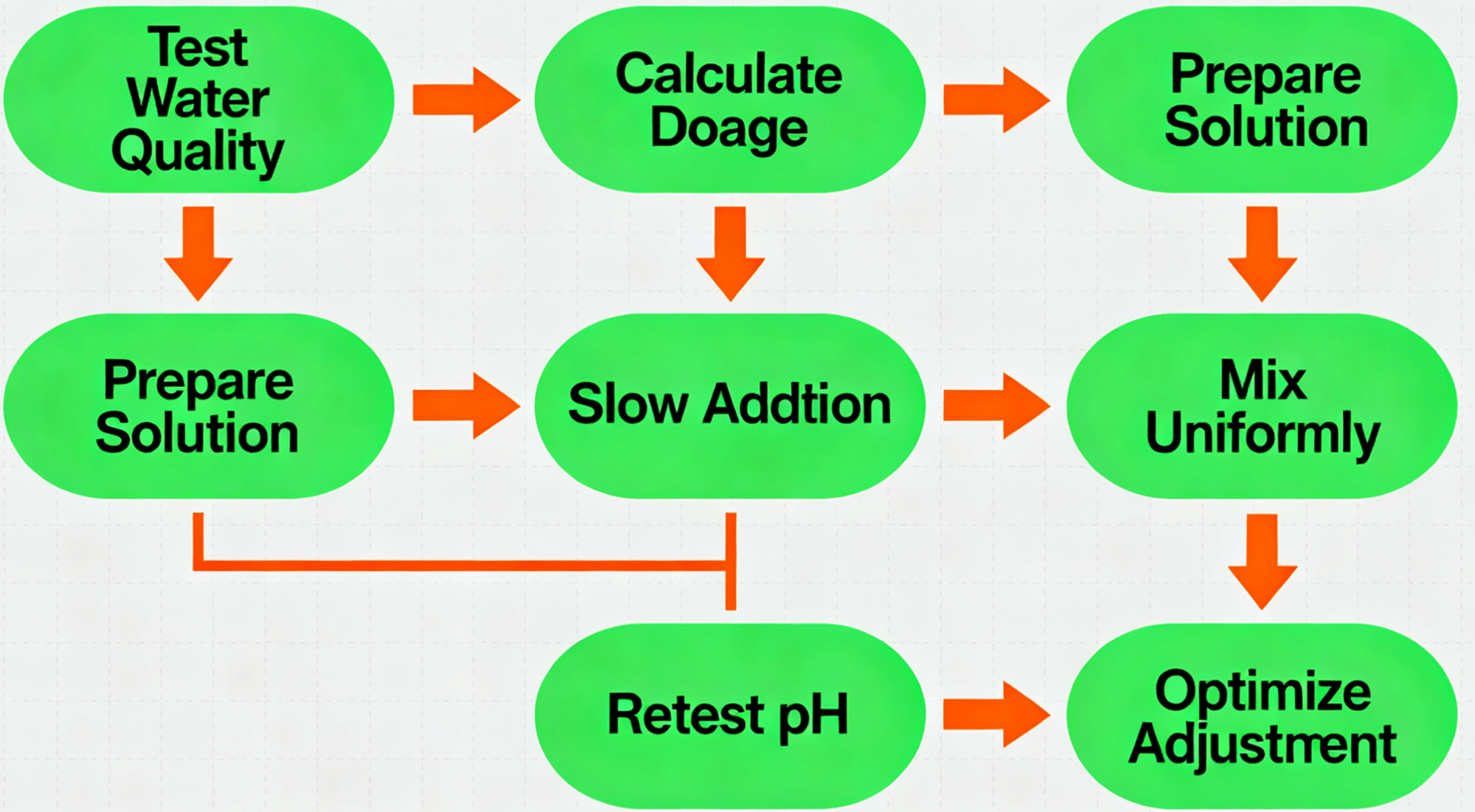
√ Avoid overdosing; split into multiple smaller additions.
√ High hardness water may form calcium carbonate precipitates.
√ Store in sealed containers to prevent moisture absorption and caking.
√ Sodium Carbonate vs Caustic Soda: Soda ash is safer but slower; caustic soda neutralizes strong acids quickly.
√ Sodium Carbonate vs Lime: Lime is cheaper but causes scaling; soda ash offers precise control.
Q1: What are the standard steps for dosing sodium carbonate?
A1: Test pH → Estimate dosage → Lab test confirmation → Prepare solution → Slow dosing → Mix → Recheck pH → Adjust if necessary.
Q2: What is the typical dosage range?
A2: Usually 50–500 mg/L, depending on acidity and water conditions.
Q3: Does sodium carbonate affect PAC or PAM efficiency?
A3: Proper dosing improves coagulation, but overdosing may hinder settling.
Q4: Is sodium carbonate safe for drinking water treatment?
A4: Yes, when applied within regulated limits, it is safe and effective.
Q5: What to do if sodium carbonate is overdosed?
A5: If pH becomes too high, add diluted acid (HCl or H₂SO₄) to bring it back to the target range.
Please contact us for free quotation by form below. We promise the quickest response within 24 hours: